Carol Smiljan/NurPhoto via Getty Images
- Novavax said Thursday it initiated a Phase 3 trial of its COVID-19 vaccine in the UK.
- The trial will enroll up to 10,000 volunteers across the UK to assess the NVX-CoV2373 vaccine candidate.
- Novavax has scaled up its manufacturing capacity in anticipation of developing a successful COVID-19 vaccine, and will be able to produce up to 2 billion annualized doses once capacity has been brought online by mid-2021.
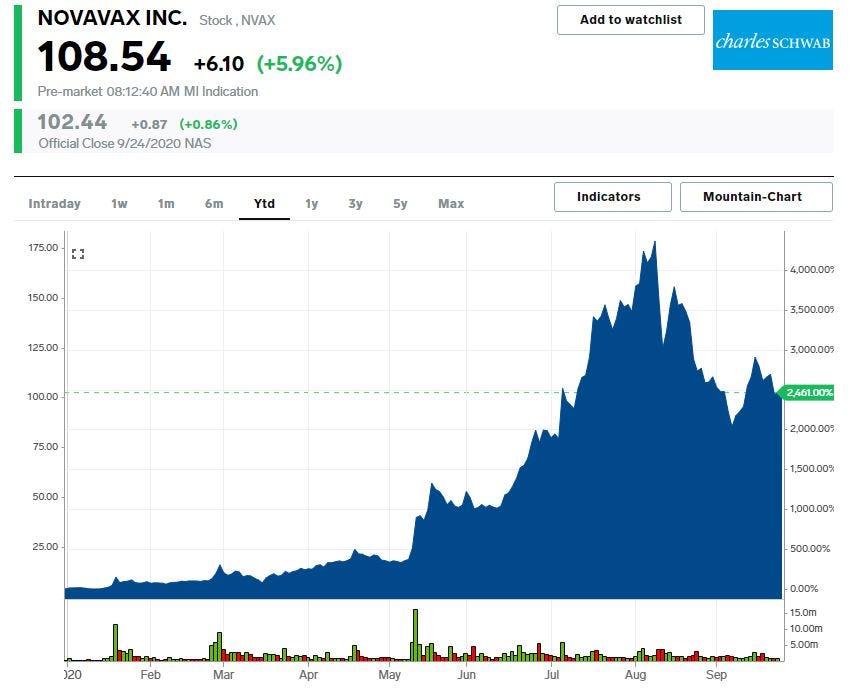
- Novavax surged as much as 15% in Friday trades.
- Visit Business Insider’s homepage for more stories.
Novavax surged on Friday after the biotechnology company said Thursday that it initiated a Phase 3 trial for its COVID-19 vaccine candidate in the UK.
Novavax, in partnership with the UK Government’s Vaccines Taskforce, will enroll up to 10,000 individuals in the trial over the next 4 to 6 weeks.
“The data from this trial is expected to support regulatory submissions for licensure in the UK, EU and other countries,” Novavax said.
Investors are likely putting more focus on successful COVID-19 vaccine developments after stocks took a hit this week, partly due to a steady uptick in COVID-19 cases across the globe.
In the scenario that Novavax succeeds in developing an effective COVID-19 vaccine candidate, it is at the ready to produce billions of doses.
According to the company, it continued to scale up manufacturing capacity, and will be able to produce up to 2 billion annualized doses by mid-2021.
The firm has partnered with Endo International to help produce the clinical and market-ready vaccines, it announced Friday.
Novavax CEO Stanley Erck said he expects a Phase 3 trial of its COVID-19 vaccine candidate "to get underway very soon" in the US.
Shares of Novavax jumped as much as 15% to $117.35 in Friday trades. Shares of Novavax are up 2,474% year-to-date as investors pin hope on Novavax to develop a successful COVID-19 vaccine.